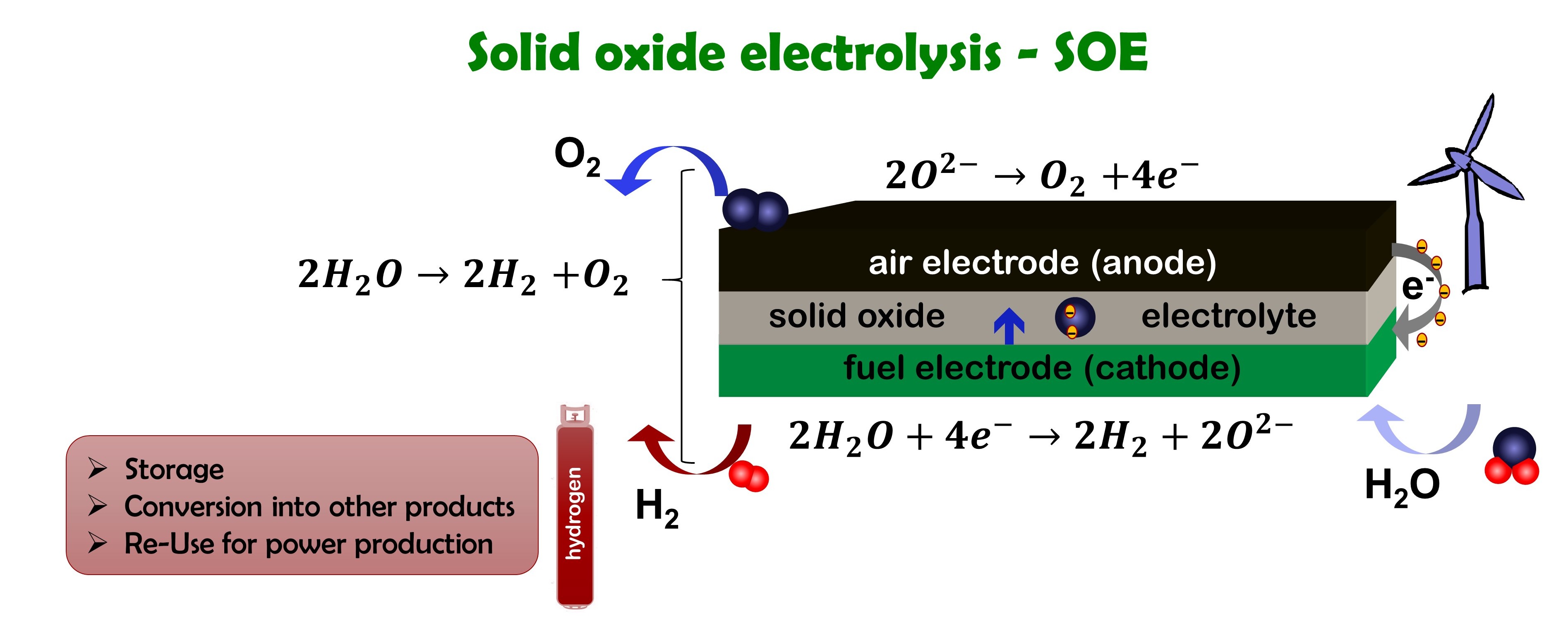
How does electrolysis work
Electrolysis is the reverse of the fuel cell. Steam is split into oxygen and hydrogen using electricity, preferable from renewable sources.
For the case of the solid oxide electrolysis (SOE), the same cell can be used as for the solid oxide fuel cell (SOFC) reaction.
- Steam is reduced to hydrogen and oxygen ions using electrons
- Oxygen ions travel through the solid oxide electrolyte
- Oxygen ions are oxidized to oxygen liberating electrons